The main qualitative characteristic of the combustion process in furnaces is its chemical completeness with a minimum excess of air, which corresponds to the highest combustion temperature. Therefore, when conducting the combustion process, it is necessary to systematically monitor the composition of combustion products and determine the coefficient of excess air and the chemical incompleteness of combustion. For this purpose, gas analysis is used, performed using gas analyzers. various systems and based on a calculation method based on the basic combustion equation.
Let's consider the equation of complete combustion. The derivation of this equation based on the balance of oxygen in combustion products was given by D. M. Khzmalyan [L. 4].
During complete combustion, the oxygen contained in the air supplied as an oxidizer is consumed for the combustion of carbon, sulfur and free hydrogen of the fuel with the formation of carbon dioxide, sulfur dioxide, water vapor, and the excess part remains in free form, i.e.:
K. =0.2"/. = C + C + C, O + ^o,- (2-42)
In the formula:
Uv and U®a - the amount of air supplied to burn 1 kg of fuel, and the amount of oxygen contained in it, m3/kg;
C. Ud*0 - respectively, oxygen consumption for the formation of carbon dioxide, sulfur dioxide and for the combustion of free hydrogen during the combustion of 1 kg of fuel, m*/kg;
U0a is the amount of free oxygen, m3/kg.
According to stoichiometric equations (2-1) and (2-2), with complete combustion of fuel, the oxygen consumption for the combustion of carbon is equal to the volume of carbon dioxide formed, and the oxygen consumption for the combustion of sulfur is equal to the volume of sulfur dioxide formed, i.e.
Ус°2 = V У50* - V
IOA уСОа» кОа
Therefore, the oxygen consumption for combustion of carbon and sulfur fuel is equal to
C+C^ae. + ‘^^o,- (2-43)
By substituting (2-43) we transform equation (2-42) to the form:
K. = 0.21V, = V + V™ + V (2-42a)
Nitrogen in the air passes into combustion products. Its amount is determined by the sum of the theoretical and excess amount of nitrogen V°Ma and
Y£°, neglecting the amount of nitrogen gasified from the fuel due to its low content in solid and liquid fuels(less
1.5-1.8%). Excess nitrogen together with free oxygen V0a
Represents excess air (a-1)У°. Comparing the total air flow for the given components of oxygen and nitrogen with the amount of dry gases determined by formula (2-12), we come to the conclusion that
^ = Us. g + Uo;°- (2-44)
After substituting (2-44) into equation (2-42a) we obtain:
Expressing all the terms included in equation (2-45) as a percentage of the volume of dry gases and simplifying it, we obtain:
21 = 1?02 + 02 + 79 . (2-46)
Expressing the volume of dry gases through the volume of triatomic gases Vc. g = = we rewrite equation (2-46) in the form of the so-called equation
Complete combustion conditions:
21 = IOg + Og + pIOg, ($2-47
In which p denotes
T/NaO T/NaO
Substituting into (2-48) according to equation (2-3) the value for 1^*°, assuming that part of the hydrogen is oxidized due to fuel oxygen, and the value for according to (2-7), the expression for p can be represented through the elemental composition of the fuel in the form:
P = 2.35<2-48а?
The physical meaning of the coefficient p (2-48) is that it shows the ratio of air oxygen consumption for the oxidation of free fuel hydrogen (i.e. fuel hydrogen, excluding its part associated with fuel oxygen) 0.01 (No. -0.126 Or) to the consumption of oxygen for the formation of triatomic gases.
Based on the known percentage of O2 in combustion products and the coefficient p, using equation (2-47), it is possible to determine the percentage of triatomic gases:
At 02-0, i.e. at a-1, the I02 content reaches its maximum value
VD “a”: = t|t. ‘ (2-50!
If the combustible components of the fuel were carbon and sulfur, and there were no oxygen and hydrogen in it, or if there was so much hydrogen that it could be oxidized due to the oxygen of the fuel, then upon complete combustion of the fuel with the theoretically required amount of air, the content of triatomic gases would be 21%, so as in this case in (2-50) according to (2-48a) p = 0.
Solid and liquid fuels usually contain less oxygen than is required for complete oxidation of hydrogen; therefore, during combustion, part of the oxygen in the air will be consumed for the oxidation of free hydrogen 0.01 (Hp-0.126 0p) of the fuel. Therefore, the I02 content in dry gases will be less than 21% and can be determined by (2-49).
As can be seen from expressions (2-48a) and (2-49), the coefficient p and the magnitude of IOg depend only on the elementary chemical composition of the fuel and therefore are important characteristics of the fuel. The values of P and I02max for some fuels are given in table. 2-3.
|
Coefficient (3 and value 102aKS for some fuels
|
The main qualitative characteristic of the combustion process in furnaces is its chemical completeness with a minimum excess of air, which corresponds to the highest combustion temperature. Therefore, when conducting the combustion process, it is necessary to systematically monitor the composition of combustion products and determine the coefficient of excess air and the chemical incompleteness of combustion. For this purpose, gas analysis is used, performed using gas analyzers of various systems and based on a calculation method based on the basic combustion equation.
Let's consider the equation of complete combustion. The derivation of this equation based on the balance of oxygen in combustion products was given by D. M. Khzmalyan [L. 4].
During complete combustion, the oxygen contained in the air supplied as an oxidizer is consumed for the combustion of carbon, sulfur and free hydrogen of the fuel with the formation of carbon dioxide, sulfur dioxide, water vapor, and the excess part remains in free form, i.e.:
K. =0.2"/. = C + C + C, O + ^o,- (2-42)
In the formula:
Uv and U®a - the amount of air supplied to burn 1 kg of fuel, and the amount of oxygen contained in it, m3/kg;
C. Ud*0 - respectively, oxygen consumption for the formation of carbon dioxide, sulfur dioxide and for the combustion of free hydrogen during the combustion of 1 kg of fuel, m*/kg;
U0a is the amount of free oxygen, m3/kg.
According to stoichiometric equations (2-1) and (2-2), with complete combustion of fuel, the oxygen consumption for the combustion of carbon is equal to the volume of carbon dioxide formed, and the oxygen consumption for the combustion of sulfur is equal to the volume of sulfur dioxide formed, i.e.
Ус°2 = V У50* - V
IOA уСОа» кОа
Therefore, the oxygen consumption for combustion of carbon and sulfur fuel is equal to
C+C^ae. + "^^o,- (2-43)
By substituting (2-43) we transform equation (2-42) to the form:
K. = 0.21V, = V + V™ + V (2-42a)
Nitrogen in the air passes into combustion products. Its amount is determined by the sum of the theoretical and excess amount of nitrogen V°Ma and
Y£°, neglecting the amount of nitrogen gasified from the fuel due to its low content in solid and liquid fuels (less
1.5-1.8%). Excess nitrogen together with free oxygen V0a
Represents excess air (a-1)У°. Comparing the total air flow for the given components of oxygen and nitrogen with the amount of dry gases determined by formula (2-12), we come to the conclusion that
^ = Us. g + Uo;°- (2-44)
After substituting (2-44) into equation (2-42a) we obtain:
Expressing all the terms included in equation (2-45) as a percentage of the volume of dry gases and simplifying it, we obtain:
21 = 1?02 + 02 + 79 . (2-46)
Expressing the volume of dry gases through the volume of triatomic gases Vc. g = = we rewrite equation (2-46) in the form of the so-called equation
Complete combustion conditions:
21 = IOg + Og + pIOg, ($2-47
In which p denotes
T/NaO T/NaO
Substituting into (2-48) according to equation (2-3) the value for 1^*°, assuming that part of the hydrogen is oxidized due to fuel oxygen, and the value for according to (2-7), the expression for p can be represented through the elemental composition of the fuel in the form:
P = 2.35<2-48а?
The physical meaning of the coefficient p (2-48) is that it shows the ratio of air oxygen consumption for the oxidation of free fuel hydrogen (i.e. fuel hydrogen, excluding its part associated with fuel oxygen) 0.01 (No. -0.126 Or) to the consumption of oxygen for the formation of triatomic gases.
Based on the known percentage of O2 in combustion products and the coefficient p, using equation (2-47), it is possible to determine the percentage of triatomic gases:
At 02-0, i.e. at a-1, the I02 content reaches its maximum value
VD “a”: = t|t. "(2-50!
If the combustible components of the fuel were carbon and sulfur, and there were no oxygen and hydrogen in it, or if there was so much hydrogen that it could be oxidized due to the oxygen of the fuel, then upon complete combustion of the fuel with the theoretically required amount of air, the content of triatomic gases would be 21%, so as in this case in (2-50) according to (2-48a) p = 0.
Solid and liquid fuels usually contain less oxygen than is required for complete oxidation of hydrogen; therefore, during combustion, part of the oxygen in the air will be consumed for the oxidation of free hydrogen 0.01 (Hp-0.126 0p) of the fuel. Therefore, the I02 content in dry gases will be less than 21% and can be determined by (2-49).
As can be seen from expressions (2-48a) and (2-49), the coefficient p and the value of IOg depend only on the elementary chemical composition of the fuel and therefore are important characteristics of the fuel. The values of P and I02max for some fuels are given in table. 2-3.
|
Coefficient (3 and value 102aKS for some fuels
|
Combustion of fuel in an engine cylinder is a complex chemical process. Omitting all intermediate stages of the combustion process, we will consider the final chemical reactions of the elements that make up the fuel with oxygen in the air.
Chemical reactions during complete combustion of liquid fuel. The elemental composition of fuels is determined using equation (36).
With complete combustion of fuel, it is assumed that as a result of reactions of carbon and hydrogen with atmospheric oxygen, carbon dioxide and water vapor are formed, respectively. In this case, the oxidation of carbon and hydrogen of the fuel corresponds to the chemical equations:
When calculating the initial and final reaction products in mass units, we obtain: for C kg I C I

When calculated in kmol

From equations (40) and (41) it is clear that as a result of the reaction of carbon with oxygen, the volume of moles of the final products of the CO2 reaction is equal to the volume of oxygen participating in the reaction. The reactions of hydrogen with oxygen lead to a twofold increase in the volume (number of moles) of water vapor compared to the oxygen consumed.
Determination of the theoretically required amount of air during complete combustion of liquid fuel. The smallest amount of oxygen O0 that needs to be supplied from outside to the fuel for its complete oxidation is called the theoretically required amount of oxygen. From equations (38) and (39) it follows that for complete combustion of 1 kg of fuel, the following amount of oxygen is needed when calculating:
![]()
or according to equations (40) and (41) when calculating in kmol
![]()
In internal combustion engines, the oxygen necessary for combustion is contained in the air, which is introduced into the cylinder during the intake process. Considering that oxygen in the air contains approximately 23% by mass and 21% by volume, we obtain, accordingly, the theoretically required amount of air for combustion
1 kg of fuel in kg:
![]()
or in kmol
![]()
hence:
![]()
for combustion of the stoichiometric mixture composition can be found through fuel characteristic 6, which is determined by the formula
The characteristics of fuel p" during its combustion in atmospheric air depend on the elemental composition of the fuel and the amount of oxygen in the air.
After some transformations, formula (45) when calculating
![]()
(in kmol) will take the form
are given in table. 5.
" moles 02, and as a result m/z moles I20 is formed. Then, taking into account the presence of oxygen O2 in a given gas, the oxidation reaction of the component is expressed by the equation

with oxygen based on formula (49) has the form
will be determined from the expression
Volume fractions of individual components in gaseous fuel.
Excess air coefficient. In a car engine, depending on the type of mixture formation, the conditions of ignition and combustion of fuel and the operating mode, the amount of air actually consumed may be greater than, equal to, or less than theoretically required for complete combustion.
in kmol) to the amount of air theoretically required for the combustion of 1 kg of fuel is called the excess air coefficient and is denoted by a:
![]()
(excess oxygen), the mixture is called lean.
due to lack of oxygen
In diesel engines in which high-quality regulation is used, the coefficient a varies widely depending on the load (from 5 or more at low load to 1.41.25 at full load). In Fig. Figure 18 shows the dependence of coefficient a on engine load.
xg is often used in the analysis of engine operating process and is called the air-fuel ratio.
(complete combustion). In a spark ignition engine, air and fuel enter the cylinder as a combustible mixture during the intake process. With complete combustion of 1 kg of fuel, the total amount of combustible mixture (in kmol), consisting of fuel vapor and air,
![]()
where rt is the molecular weight of the fuel (see Table 5).
In a diesel engine, the air-fuel mixture is formed in the combustion chamber during fuel injection at the end of the compression process and during the combustion process. As a result of this, and also because of the small volume occupied, the molecular mass of the fuel is not taken into account,
For gaseous fuel (in kmol or m3)
For any fuel, mixture mass (in kg)

The amount of individual components of combustion products (in kmol) is determined by the following equations:

Mass of oxygen that took part in the reaction, kmol.
we get (in kmol)

After substituting expressions (58), (60) and (62) into equation (57), we find:
its value from expression (45), we will have (in kmol)
Let us determine the amount of combustion products (in kmol) through the characteristics of the fuel. From formulas (58), (59), (61) and (62) we have
After appropriate transformations we get
![]()
![]()
amount of combustion products (in kmol)
Accordingly, the masses of excess nitrogen and oxygen in combustion products depend on the excess air ratio.
Mass of combustion products (in kg) during combustion of 1 kg of liquid fuel
Let us determine the amount of combustion products during the combustion of gaseous fuel. For 1 mole (or 1 m3) of gaseous fuel we have the number of individual components (in moles or m3)

where N2 is the amount of nitrogen in the fuel, mol or m3.
When 1 mole or 1 m3 of gaseous fuel is burned, the amount of combustion products (in moles or m3)
from formula (50), then
where Mo is in mole or m3.
Considering that
we get (in moles or m3)
from equation (74) we have
shows that the ratio of the number of moles of hydrogen and carbon monoxide is approximately constant for a given fuel and does not depend on the value of a. Let us denote this relationship by
The chemical reaction of carbon with oxygen during incomplete combustion has the form
![]()
the volume of combustion products increases 2 times compared to the volume of oxygen that took part in combustion.
) quantity of products
Combustion value (in kmol)
The amount of water vapor in combustion products in the case of incomplete combustion is determined from the equation
Amount of free hydrogen (in kmol) in combustion products
Total amount of water vapor and hydrogen in combustion products (in kmol)
Taking into account the nitrogen contained in the air, the total amount of combustion products from equations (82) and (85) (in kmol)
through the fuel characteristic [Eq.
The amount of oxygen participating in the reaction required for the combustion of carbon
![]()
carbon in CO
hydrogen
![]()
The total amount of oxygen involved in the reaction is
From equations (82), (85) and (79) we have

After substituting expressions (92) and (93) into equation (91), we obtain
The amount of each component (in kmol) included in the combustion products is determined by the following formulas, obtained respectively from expressions (79), (92), (93) and (95):
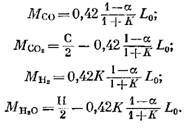
Nitrogen amount
![]()
Soot particles are solid filtrate consisting mainly of solid carbon C.
the thermal effect of the reaction is reduced as a result of the formation of CO from part of the carbon. The presence of these components is extremely undesirable, as they have toxic properties. When these components are removed from the engine cylinder with exhaust gases, they pollute the air and have a harmful effect on human health. Therefore, recently special attention has been paid to the neutralization of exhaust gases emitted into the atmosphere. The toxic components of combustion products also include lead oxides formed during the combustion of leaded gasoline (see Table 2).
aldehydes and soot result from incomplete combustion and thermal decomposition of hydrocarbons even when there is excess oxygen. The amount of these components depends on the nature of the intermediate chemical reactions.
However, its concentration is relatively low.
in combustion products is explained by the presence of near-wall zones “in the combustion chamber, where due to the contact of the charge with the walls, which have relatively low temperatures, the flame is extinguished.
Aldehydes are produced during the period when the oxidation process occurs at low temperatures. This phenomenon is observed during start-up, as well as during operating modes in those areas where the burning mixture is cooled by relatively cold surfaces that limit the combustion chamber. In a diesel engine, where fuel injection begins immediately before combustion begins, aldehydes are formed during so-called pre-flame reactions that occur during the preparation of the air-fuel mixture for combustion (see Chapter VI). The operation of a diesel engine with a very lean mixture, which is typical for low loads, as well as the combustion of the last portion of fuel in gasoline engines, when a special method of organizing the combustion process (layer-by-layer mixture formation) is used, leads to the formation of aldehydes.
in different areas of the chamber
Along with combustion, the fuel breaks down and carbon (soot) is released. In carburetor engines, the composition of the mixture is homogeneous (homogeneous), and soot is formed in almost insignificant quantities during normal engine operation.
Nitrogen oxides are produced in the presence of atomic oxygen in those areas of the combustion chamber in which the temperature rises sharply as a result of the chemical reaction of oxidation of fuel hydrocarbons. The amount of nitrogen oxide formed depends on the nitrogen and oxygen content in the combustion products.
is determined by the conditions of exchange diffusion of combustion products with atmospheric air.
in combustion products when a spark-ignition engine is running without load and idling (GOST 1653370) and on the smoke content in diesel exhaust gases (GOST 1902573).
Composition of combustion products. Depending on what requirements are set when determining the composition of combustion products, the appropriate equipment and analysis technique are selected. Special literature is devoted to the equipment and methods of analyzing gas samples.
In Fig. Figure 19 shows curves for the content of combustion products in the exhaust gases of a diesel engine and a carburetor engine depending on a. The change in coefficient a depends on the engine load.
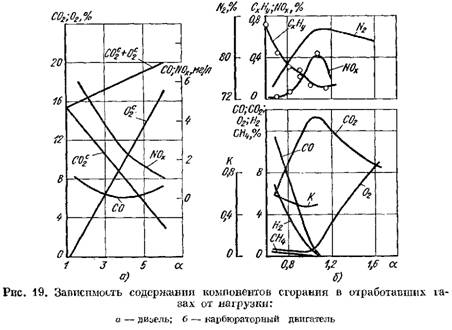
in terms of dry weight (Fig. 19, a)
increases.
2 increases sharply and the combustion products contain a small amount of oxygen that did not participate in combustion.
![]() when the combustion process deteriorates.
when the combustion process deteriorates.
Combustible fuel substances interact with air oxygen in a certain quantitative ratio. Air consumption for combustion and the amount of fuel combustion products are calculated using stoichiometric combustion equations, which are written for 1 km for each combustible component.
Theoretical and actual combustion air consumption and the amount of fuel combustion products. The stoichiometric equations for the combustion of combustible components of solid and liquid fuels have the form:
carbon C + O 2 = CO 2:
12 kg C + 32 kg O 2 = 44 KG CO 2;
1 kg C + (32: 12) kg O 2 = (44: 12) kg CO 2 (18.21)
sulfur S + O 2 = SO 2:
32 kg S + 32 kg O 2 = 64 kg SO 2;
1 kg S + 1 kg O 2 = 2 kg SO 2; (18.22)
hydrogen 2H 2 + O 2 = 2H 2 O:
4 kg H 2 + 32 kg O 2 = 36 kg H 2 O;
1 kg H 2 + 8 kg O 2 = 9 kg H 2 (18.23)
The fuel contains C p /100 kg of carbon, S p / l 100 kg of volatile sulfur, H p /100 kg of hydrogen and O p /100 kg of oxygen. Consequently, the total consumption of oxygen required for the combustion of 1 kg of fuel, according to the stoichiometric equations, will be equal to:
The mass fraction of oxygen in air is 0.232. Then the mass amount of air is determined from the formula:

Under normal conditions, air density p0 = 1.293 kg/m3.
Combustion air consumption and the amount of fuel combustion products can be easily calculated as:
V 0 = M 0 /1.293 m 3 air/kg fuel. (18.26)
Thus,
V 0 = 0.0889 (C p + 0.375S p / l) + 0.265H p - 0.033O p (18.27)
For gaseous fuel, the consumption V0 is determined based on the volume fractions of combustible components included in the gas using stoichiometric reactions:
H 2 + 0.5O 2 = H 2 O;
CO + 0.5O 2 = CO 2;
CH 4 + 2O 2 = CO 2 + 2H 2 O;
H 2 S + 1.5 O 2 = SO 2 + H 2 O. (18.28)
The theoretical amount of air, m 3 / m 3, required for gas combustion is determined by the formula:

The volume concentration of the components, %, is substituted into equation (18.29). If there is no data on the concentration of unsaturated hydrocarbons, they are considered to consist of C 2 H 4.
The amount of air V n, calculated using the stoichiometric equations (18.27) and (18.29), is called theoretically necessary, i.e. the value V 0 is the minimum amount of air required to ensure complete combustion of 1 kg (1 m 3) of fuel, provided that all the oxygen in it and the oxygen contained in the fuel will be used during combustion.
Due to certain difficulties in organizing the process of complete mixing of fuel with air in the working volume of the furnaces, areas may appear where a local deficiency or excess of oxidizer will be felt. As a result, the quality and consumption of combustion air and the quantity of fuel combustion products deteriorate. Therefore, in real conditions, air for fuel combustion is supplied in larger quantities compared to its theoretical amount V 0 . The ratio of the actual amount of air supplied to the firebox to the theoretically required one is called the excess air coefficient:
α = V d /V 0.(18.30)
When designing and thermally calculating furnaces or other combustion chambers, the value of a is chosen depending on the type of fuel burned, the combustion method and the design features of the combustion chambers. The a value ranges from 1.02 to 1.5.
Composition and quantity of products of complete combustion of fuel. The products of complete combustion of fuel at α = 1 contain: dry (non-condensing in the boiler unit) triatomic gases CO 2 and SO 2;
H 2 O - water vapor obtained from the combustion of hydrogen; N 2 - fuel nitrogen and nitrogen contained in the theoretically required amount of air.
In addition, the combustion products include water vapor resulting from the evaporation of fuel moisture, steam introduced into the furnace with moist air, and steam sometimes used when burning fuel oil for atomization. When the temperature of the combustion products is below the dew point temperature, water vapor condenses. With complete combustion with α = 1, the combustion products will contain only CO 2, SO 2, H 2 O and N 2; if α > 1, then they will also contain excess air, i.e., additional amounts of oxygen and nitrogen.
The percentage content of the corresponding gases by volume will be denoted by CO 2, N 2, SO 2, etc., and by V co2, V so2, V n2, etc. - their volumes obtained by burning 1 kg (1 m3) of fuel, reduced to normal conditions (index 0 indicates that calculations are made at α = 1). Then we get:
CO 2 + SO 2 + N 0 / 2 + H 2 O 2 = 100%
V 0 / r = V co2 + V S2O + V 0 / N2 + V 0 H2O (18.31)
Where V o / r is the total volume of combustion products, reduced to normal conditions, m 3 / kg.
To simplify calculations, the volumes of dry triatomic gases are calculated together and their sum is conventionally designated by the symbol RO 2, i.e.
V ro2 = V co2 + V so2 (18.32)
The sum of the first three components in equality (18.31) represents the volume of dry gases V с.p and, therefore,
V o r = V o c.g + V 0 H2O (18.33)
where V 0 c.r = V ro2 + V 0 N2
The quantities V 0 N2, V 0 H2O, V 0 c.r, V 0 and V ro2 are the theoretical volumes of gases when burning 1 kg of solid or liquid fuel. In accordance with equations (18.21) and (18.22), the mass of triatomic gases is equal to:
The densities of carbon dioxide and sulfur dioxide, reduced to normal conditions, are respectively equal to p co2 = 1.964 kg/m 3 and P so2 = 2.86 kg/m 3. Then the volume of triatomic gases V RO2 can be determined by the formula:

The theoretical volume of water vapor formed during the combustion of hydrogen V r H2O is determined in accordance with equation (18.23). To this volume it is necessary to add the volume of steam formed during the evaporation of fuel moisture V r H2O. the volume of nozzle steam V f H2O and the volume of water vapor contained in the air V in H2O then: 
where 0.805 is the density of water vapor under normal physical conditions, kg/m 3 ; W f - nozzle steam consumption (assumed W f = 0.3 ÷ 0.35 kg/kg),
The total theoretical volume of water vapor is determined by the formula:
V 0 H 2 O = 0.111H p + 0.0124 W p + 1.24 W f + 0.0161 V 0 (18.41)
The theoretical volume of nitrogen (1 m3 per 1 kg of fuel) at α = 1 consists of air nitrogen and fuel nitrogen, i.e.
where p N2 = 1.25 - nitrogen density, kg/m 3.
When the excess air coefficient α > 1, the composition of the combustion products will additionally include excess air and water vapor introduced with this air. Volumes of combustion products at
α = 1 are called real volumes.
Actual volumes will be:

The value of V RO2 does not depend on the value of the excess air coefficient.
Theoretical volumes of combustion products of 1 m 3 of gaseous fuel are calculated based on stoichiometric reactions (18.28). With complete combustion of the combustible components of gaseous fuel CO, H 2 and C m H n, carbon dioxide CO 2 and water vapor are formed. When hydrogen sulfide H 2 S is burned, in addition to water vapor, sulfur dioxide SO 2 is formed.
Volume of triatomic gases, m 3 / m 3,

where a is the moisture content of the gas, g/m3.
The volume of nitrogen, dry gases and the total volume of combustion products, as well as for liquid and solid fuels, are determined using formulas (18.42), (18.33) and (18.31).
For α > 1, the actual volume of water vapor, dry gas and the total volume of combustion products are found using formulas (18.43), (18.45) and (18.46). If the composition of unsaturated hydrocarbons CnH2n included in the gas is unknown, and their total content does not exceed 3%, then in the calculation they are taken into account as C2H4.
Combustion calculations based on gas analysis results. Gas analysis of combustion products is intended to control the quality (completeness) of fuel combustion. For this purpose, chemical gas analyzers such as VTI and GPC - 3 are used. The principle of their operation is based on the selective absorption of components included in combustion products by chemical reagents that fill the absorption columns of the gas analyzer. For example, to absorb RO 2 a KOH solution is used, and to absorb O 2 an alkaline solution of pyrogallol C 6 H 3 (OH) 3 is used.
Currently, chromatographic gas analysis is widely used, based on physical methods for separating gas mixtures into their constituent components. The principle of operation of the chromatograph is based on the difference in the adsorption properties of various gases as they pass through a layer of sorbent (silica gel).
Calculations based on the results of gas analysis are made for the volume of dry gases.
Determination of carbon monoxide during combustion of solid and liquid fuels. When fuel is incompletely burned, the combustion products always contain some amount of carbon monoxide CO. The equation for the composition of dry combustion products has the form
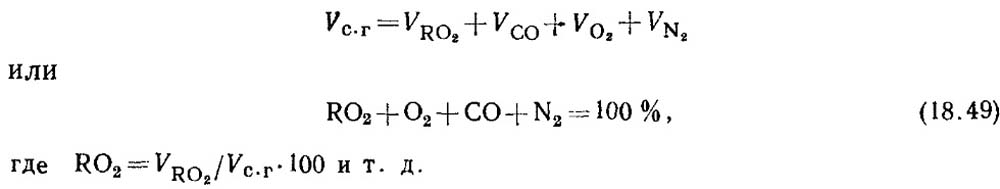
Based on the results of gas analysis, the percentage of RO 2 and O 2 in combustion products is determined.
Determination of CO using the absorption method is undesirable due to the rather large error of the method. Therefore, the CO content in gases is determined by calculation.
With complete combustion of pure carbon and α = 1, the combustion products contain CO 2 and N 2, with COmax2 = RO max2 = 21%. In addition to carbon, the combustible mass of fossil fuels always contains some amount of hydrogen. Therefore, the concentration of RO max 2 in dry combustion products is always less than 21%, i.e., with a decrease in the carbon and sulfur content in the fuel, the value of RO max 2 also decreases. There is a certain dimensionless quantity that can be used to establish the relationship between the elemental composition of the fuel and the content of three atomic gases in the dry combustion products. This value is called the fuel characteristic, and is denoted by the letter β.

The values of RO max 2 and β for each type of fuel of a certain composition are constant (Table 18.4).
Table 18.4. RO max 2 AND β values for some fuels.

As the excess air coefficient β increases above unity, free oxygen and RO 2 will appear in the dry products of complete combustion as a result of excess air< RO мак с 2 . При известном значении α содержание RО 2 можно приближенно определять по эмпирической формуле:
RO 2 = RO max 2 / α (18.52)
In the specialized literature, the so-called equation for complete combustion of fuel is derived:
RO 2 + O 2 = 21 β RO 2. (18.53)
If the right and left sides of equation (18.53) are not equal to each other, then there is no complete combustion, and in this case the difference (21 - βPRO 2 l) - (RO 2 + O 2) = z is called the characteristic of incomplete combustion of the fuel.
The equation for incomplete combustion of fuel is written as follows:
21 - β RO 2 = RO 2 + O 2 + (0.605 + β) CO.(18.53")
When using the chromatographic method of gas analysis, there is no need to calculate CO using formula (18.54), since the carbon monoxide content can be determined directly on the chromatograph.
Air consumption for combustion and the amount of fuel combustion products during incomplete combustion. The volume of dry combustion products is determined from the results of gas analysis in accordance with equation (18.49) as follows. Percentage of RO 2 in gases:

Determination of flammable CO constituents CO, CH 4 and H 2 during incomplete combustion of gaseous fuel. The composition equation for dry combustion products can be written as follows:
Using the chromatographic method of gas analysis, it is possible to determine all components of the combustible part of the combustion products CO, CH 4 and H 2.
If the results of the analysis are known only for RO 2 and O 2, then to determine CO in gases, the value of the fuel characteristic β is taken according to reference data or, if the composition of the initial combustible gas is known, β is determined by calculation with mandatory consideration of the CO t 2 content in the gas:
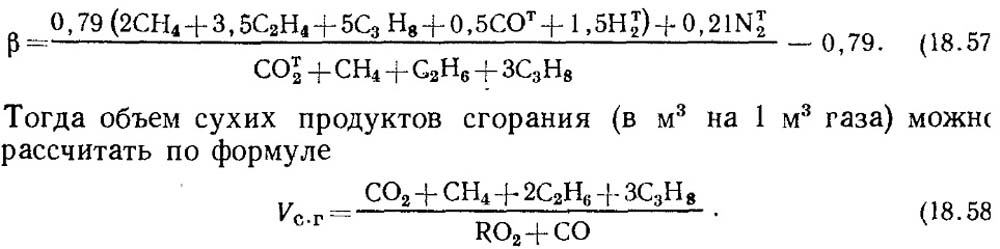
It should be noted that for artificial gases that contain a large amount of CO t 2, the fuel characteristic β may be negative.
Determination of excess air coefficient. The excess air coefficient is determined from gas analysis data of dry combustion products. For the case of complete combustion, when there are no flammable components CO, CH 4, H 2 in the combustion products

In case of incomplete combustion

With complete combustion of fuel and a known value of RO max 2, formula (18.52) can be used for determination.
Example. Determine the lower and higher heat of combustion of natural Saratov (Elshan) gas having the following volumetric composition,%: CH 4 - 94, C 2 H 6 - 1.8, C 3 H 8 - 0.4, C 4 H 10 - 0, 1, C 5 H 12 - 0.1,
CO 2 - 0.1, N 2 - 3.5.
Solution: 1. The lowest calorific value, kJ/m 3, is: methane CH 4 - 35.8 × 10 3, ethane C 2 H 4 - 64.6 × 10 3, propane C 3 H 8 - 91.5 × 10 3, butane C 4 H 10 - 119.0 × 10 3, pentane C 5 H 12 - 146.5 × 10 3.
Using formula (18.6) we determine
Q c n = (35.8×94 + 64.6×1.8 + 91.5×0.4+ 119×0.1 + 146.5×0.1) 103×0.01 = 35,300 kJ/ m 3.
The highest calorific value, kJ/m 3, is: methane CH 4 - 39.8 × 10 3, ethane C 2 H 4 - 70 × 10 3, propane C 3 H 8 - 99.5 × 10 3, butane C 4 H 10 - 28.5 × 10 3, pentane C 8 H 12 - 157.5 × 10 3.
Using formula (18.17) we find
Q с в = (39.8 × 94 + 70 × 1.8 + 99.5 × 0.4 + 128.5 × 0.1 +157.5 × 0.01) 103 × 0.01 = 39,300 kJ /m 3.