Combustion is chemical process combination of a flammable substance with an oxidizing agent, accompanied by intense release of heat and emission of light.
The condition for combustion to occur is that the rate of heat release by a chemical reaction exceeds the rate of heat removal in environment. If this condition is met, self-heating occurs combustible mixture and the reaction rate increases. Conversely, an excess of the rate of heat removal over the rate of its release leads to attenuation of the combustion process.
There are several types of combustion:
A flash is the rapid combustion of a flammable mixture without the formation of increased gas pressure.
Fire – the occurrence of combustion from an ignition source.
Ignition is a fire accompanied by the appearance of a flame.
Spontaneous combustion is a combustion that occurs in the absence of an external ignition source.
Spontaneous combustion is spontaneous combustion accompanied by the appearance of a flame.
An explosion is an extremely rapid combustion during which energy is released and compressed gases are formed that can cause mechanical destruction.
The combustion of gases occurs in the diffusion (when oxygen penetrates into the combustion zone) and kinetic (homogeneous combustible mixture) region and can be of the nature of explosive or detonation (high speed of flame movement) combustion.
When a liquid burns, it evaporates and the steam-air mixture burns above the surface of the liquid. The determining factor is the process of liquid evaporation, which depends on its physical and chemical properties, the thermal process in it, etc. The combustion process of vapors is no different from the combustion of gases.
Combustion of solids is heterogeneous-diffusion (that is, combustion in different phases with penetration - melting, decomposition and evaporation with the release of gaseous products that form a combustible mixture with air).
Dust has an increased fire hazard. Moreover, with increasing dispersion (this is essentially saturation, the ratio of the surface area of particles to the volume they occupy) of dust, its chemical activity increases, the temperature of self-ignition decreases, which increases its fire hazard. The burning speed of highly dispersed dust approaches the burning speed of gas. Not only suspended dust, but also settled dust is explosive, since upon ignition it becomes suspended, which leads to secondary explosions.
Depending on speed chemical reaction and the formation of a flammable mixture, combustion can occur in the form of:
· smoldering - speed up to several cm/s;
· self-combustion - speed up to several m/s;
· explosion - speed several hundred m/s;
· detonation - speed up to several thousand m/s
Combustion reactions include not only the interaction reactions between combustible substances and oxygen, but also other redox reactions: the interaction of certain substances with halogens, sulfur vapor, decomposition reactions of explosives, and some endothermic compounds, for example, acetylene.
H 2 + Cl 2 = 2 HCl + Q
C 3 H 5 (NO 3) 3 = 3CO 2 +2.5H 2 O + 1.5N 2 + 0.25O2
C 2 H 2 = 2C + H 2 + Q
Classes of fires by type of combustion materials. To successfully extinguish a fire, it is necessary to use the most effective fire extinguishing agents, the choice of which must be decided almost instantly.
Right choice fire extinguishing agent will ensure rapid cessation of combustion.
This task is greatly simplified due to the introduction of fire classification. The International Standards Organization introduces 5 classes of fires (Standard 3941-77):
Class A: Hard Materials
Class B: Flammable liquids
Class C: Combustion of gases, incl. liquefied
Class D: Alkali metals (sodium, lithium, calcium, etc.)
Class E: Live electrical appliances and wiring.
Class "A" fires - combustion of solid combustible materials. Such materials include wood and wood products, fabrics, paper, rubber, some plastics and others.
These materials are extinguished mainly with water, aqueous solutions, and foam.
Fires of class "B" - combustion of liquid substances, their mixtures and compounds. This class of substances includes oil and liquid petroleum products, fats, paints, solvents and other flammable liquids.
Extinguishing such fires is carried out mainly with the help of foam by covering it with a layer of the surface of a flammable liquid, thus separating it from the combustion zone and the oxidizer. In addition, class “B” fires can be extinguished with sprayed water, powders, and carbon dioxide.
Class "C" fires - combustion gaseous substances and materials. This class of substances includes flammable gases used on sea vessels as technological supplies, as well as flammable gases transported by sea vessels as cargo (methane, hydrogen, ammonia, etc.). Extinguishing flammable gases is carried out with compact jets of water or using fire extinguishing powders.
Class "D" fires are fires involving alkali and similar metals and their compounds in contact with water. Such substances include sodium, potassium, magnesium, titanium, aluminum, etc. Heat-absorbing agents are used to extinguish such fires. fire extinguishing agents, for example, some powders that do not react with burning materials.
Class “E” fires are combustion that occurs when energized electrical equipment, conductors or electrical installations ignite. To combat such fires, fire extinguishing agents that are not conductors of electricity are used.
Types of combustion, their characteristics.
| Parameter name | Meaning |
| Article topic: | Types of combustion, their characteristics. |
| Rubric (thematic category) | Production |
Fire- ϶ᴛᴏ uncontrolled combustion outside a special fireplace, causing material damage and creating a danger to human life.
Types of combustion:
1) According to the speed of flame propagation, combustion is distinguished: 1. Normal (propagation speed of several m/s); 2. Explosive (speed hundreds of m/s); 3. Detonation (speed of thousands of m/s)
2) Taking into account the dependence on the aggregate state of flammable substances: homogeneous and heterogeneous.
If the components of the combustible mixture are pre-mixed, then kinetic combustion occurs, which is determined by the rate of the chemical reaction. If the components are not mixed, then diffusion combustion occurs, which is determined by the diffusion of oxygen to the combustible substance through the combustion products. Laminar combustion is characterized by layer-by-layer propagation of the flame front through a fresh combustible mixture. Turbulent combustion is characterized by mixing of layers and an increased burnout rate.
3) According to the combustion mode: 1. Spontaneous ignition - the spontaneous occurrence of flaming combustion of a combustible mixture preheated to the boiling temperature, which is usually called the self-ignition temperature (); 2. Propagation of the flame front through a fresh combustible mixture when it is locally ignited by an external source. Spontaneous combustion processes are divided into the following types:
Thermal as a result of prolonged action of a heat source;
Microbiological occurs as a result of increased temperature and humidity due to the vital activity of organisms (sawdust, grain, peat);
A chemical reaction occurs when substances interact with each other or with oxygen.
According to the second mode, the following types of combustion are distinguished:
- Flash– rapid combustion of a gas-vapor-air mixture over the surface of a condensed substance, accompanied by a short-term visible glow without the formation of increased gas pressure. Characterized by flash point. Flash point is the lowest temperature at which vapors and gases are formed above the surface of a condensed substance that can flare up in the air from an ignition source.
- Fire– the occurrence of combustion from the ignition source;
- Ignition– flaming combustion of a substance that continues after the ignition source is removed. Characterized by ignition temperature. Ignition temperature is the lowest temperature at which stable flame combustion occurs above the surface of the condensed substance.
- Explosion– extremely rapid combustion during which energy is released and compressed gases are formed that can cause mechanical destruction. Characterized by maximum explosion pressure.
Combustion should be: 1. Complete – with excess oxygen. Combustion products are water vapor and carbon dioxide. 2. Incomplete - there is not enough oxidizing agent, and carbon monoxide is formed.
Dangerous factors of fire include: - open fire, - sparks, - increased air temperature, - toxic combustion products, - reduced oxygen concentration.
Types of combustion, their characteristics. - concept and types. Classification and features of the category "Types of combustion, their characteristics." 2014, 2015.
Topic 4. TYPES OF COMBUSTION.
According to various characteristics and characteristics, combustion processes can be divided into the following types:
According to the state of aggregation of a flammable substance:
Combustion of gases;
Combustion of liquids and melting solids;
Combustion of non-melting solid dust-like and compact substances.
According to the phase composition of the components:
Heterogeneous combustion;
Combustion of explosives.
According to the preparedness of the combustible mixture:
Diffusion combustion (fire);
Kinetic combustion (explosion).
According to the dynamics of the flame front:
Stationary;
Unsteady.
According to the nature of gas movement:
Laminar;
Turbulent.
According to the degree of combustion of the flammable substance:
Incomplete.
According to the speed of flame spread:
Normal;
Deflagration;
Detonation.
Let's take a closer look at these types.
4.1. Combustion of gaseous, liquid and solid substances.
Depending on the state of aggregation of the combustible substance, combustion of gases, liquids, dusty and compact solids is distinguished.
According to GOST 12.1.044-89:
1. Gases are substances critical temperature which are less than 50 o C. Tcr is the minimum heating temperature of 1 mole of a substance in a closed vessel, at which it completely turns into steam (see § 2.3).
2. Liquids are substances with a melting point (dropping point) of less than 50 o C (see § 2.5).
3. Solids are substances with a melting point (dropping point) of more than 50 0 C.
4. Dusts are crushed solids with a particle size of less than 0.85 mm.
The area in which a chemical reaction occurs in a flammable mixture, i.e. combustion is called a flame front.
Let us consider combustion processes in air environment with examples.
Combustion of gases in a gas burner. There are 3 flame zones observed here (Fig. 12):
Rice. 12. Scheme of gas combustion: 1 – transparent cone – this is the initial gas that is heated (to the auto-ignition temperature); 2 – luminous zone of the flame front; 3 – combustion products (they are almost invisible during complete combustion of gases and, especially during the combustion of hydrogen, when soot is not formed).
The width of the flame front in gas mixtures is tens of fractions of a millimeter.
Combustion of liquids in an open vessel. When burning in an open vessel, there are 4 zones (Fig. 13):

Rice. 13. Liquid combustion: 1 – liquid; 2 – liquid vapors (dark areas); 3 – flame front; 4 – combustion products (smoke).
The width of the flame front in this case is larger, i.e. the reaction proceeds more slowly.
Combustion of melting solids. Consider the burning of a candle. IN in this case 6 zones are observed (Fig. 14):

Rice. 14. Candle burning: 1 – hard wax; 2 – molten (liquid) wax; 3 – dark transparent vapor layer; 4 – flame front; 5 – combustion products (smoke); 6 – wick.
The burning wick serves to stabilize the combustion. Liquid is absorbed into it, rises through it, evaporates and burns. The width of the flame front increases, which increases the luminosity area, since more complex hydrocarbons are used, which, when evaporated, disintegrate and then react.
Combustion of non-melting solids. We will consider this type of combustion using the example of the combustion of a match and a cigarette (Fig. 15 and 16).
There are also 5 sections here:
Rice. 15. Burning a match: 1 – fresh wood; 2 – charred wood; 3 – gases (gasified or evaporated volatile substances) – this is a darkish transparent zone; 4 – flame front; 5 – combustion products (smoke).
It can be seen that the burnt area of the match is much thinner and has a black color. This means that part of the match has become charred, i.e. the non-volatile part remained, and the volatile part evaporated and burned. The burning rate of coal is much slower than that of gases, so it does not have time to burn out completely.
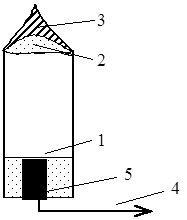
Fig. 16. Cigarette burning: 1 – initial tobacco mixture; 2 – smoldering section without a flame front; 3 – smoke, i.e. product of burnt particles; 4 – smoke drawn into the lungs, which is mainly gasified products; 5 – resin condensed on the filter.
The flameless thermal-oxidative decomposition of a substance is called smoldering. It occurs when there is insufficient diffusion of oxygen into the combustion zone and can occur even with a very small amount of oxygen (1-2%). The smoke is bluish, not black. This means there are more gasified rather than burnt substances in it.
The surface of the ash is almost white. This means that with a sufficient supply of oxygen, complete combustion occurs. But inside and at the border of the burning layer with fresh layers there is a black substance. This indicates incomplete combustion of charred particles. By the way, vapors of evaporated resinous substances condense on the filter.
A similar type of combustion is observed when burning coke, i.e. coal from which volatile substances (gases, resins) have been removed, or graphite.
Thus, the combustion process of gases, liquids and most solids occurs in gaseous form and is accompanied by a flame. Some solid substances, including those with a tendency to spontaneous combustion, burn as smoldering on the surface and inside the material.
Combustion of dusty substances. The dust layer burns in the same way as in a compact state, only the burning rate increases due to an increase in the surface of contact with air.
The combustion of dusty substances in the form of an air suspension (dust cloud) can occur in the form of sparks, i.e. combustion of individual particles, in the case of a low content of volatile substances that are not capable of forming a sufficient amount of gases during evaporation for a single flame front.
If a sufficient amount of gasified volatile substances is formed, flaming combustion occurs.
Combustion of explosives. TO this species This includes the combustion of explosives and gunpowder, the so-called condensed substances, which already contain chemically or mechanically bound fuel and oxidizer. For example: in trinitrotoluene (TNT) C 7 H 5 O 6 N 3 × C 7 H 5 × 3NO 2 the oxidizing agents are O 2 and NO 2; gunpowder contains sulfur, saltpeter, coal; The homemade explosive consists of aluminum powder and ammonium nitrate, and the binder is solar oil.
4.2. Homogeneous and heterogeneous combustion.
Based on the examples considered, depending on the state of aggregation of the mixture of fuel and oxidizer, i.e. depending on the number of phases in the mixture, there are:
1. Homogeneous combustion gases and vapors of flammable substances in a gaseous oxidizer environment. Thus, the combustion reaction occurs in a system consisting of one phase (aggregate state).
2. Heterogeneous combustion solid flammable substances in a gaseous oxidizer environment. In this case, the reaction occurs at the interface, while a homogeneous reaction occurs throughout the volume.
This is the combustion of metals, graphite, i.e. practically non-volatile materials. Many gas reactions are of a homogeneous-heterogeneous nature, when the possibility of a homogeneous reaction occurring is due to the origin of a simultaneously heterogeneous reaction.
The combustion of all liquid and many solid substances, from which vapors or gases (volatile substances) are released, occurs in the gas phase. Solid and liquid phases play the role of reservoirs of reacting products.
For example, the heterogeneous reaction of spontaneous combustion of coal passes into the homogeneous phase of combustion of volatile substances. The coke residue burns heterogeneously.
4.3. Diffusion and kinetic combustion.
Based on the degree of preparation of the combustible mixture, diffusion and kinetic combustion are distinguished.
The types of combustion considered (except for explosives) relate to diffusion combustion. Flame, i.e. The combustion zone of a mixture of fuel and air must be constantly fed with fuel and oxygen in order to ensure stability. The supply of combustible gas depends only on the speed of its supply to the combustion zone. The rate of entry of flammable liquid depends on the intensity of its evaporation, i.e. on the vapor pressure above the surface of the liquid, and, consequently, on the temperature of the liquid. Ignition temperature is the lowest temperature of a liquid at which the flame above its surface will not go out.
The combustion of solids differs from the combustion of gases by the presence of a stage of decomposition and gasification with subsequent ignition of volatile pyrolysis products.
Pyrolysis is the heating of organic substances to high temperatures without air access. In this case, the decomposition, or splitting, of complex compounds into simpler ones occurs (coking of coal, cracking of oil, dry distillation of wood). Therefore, the combustion of a solid combustible substance into a combustion product is not concentrated only in the flame zone, but has a multi-stage character.
Heating the solid phase causes decomposition and the release of gases, which ignite and burn. The heat from the torch heats the solid phase, causing it to gasify and the process repeats, thus maintaining combustion.
Combustion model solid assumes the presence of the following phases (Fig. 17):
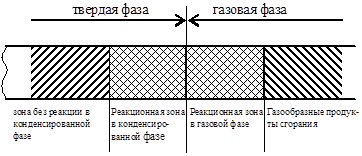
Rice. 17. Combustion model
solid matter.
Warming up the solid phase. For melting substances, melting occurs in this zone. The thickness of the zone depends on the conductivity temperature of the substance;
Pyrolysis, or reaction zone in the solid phase, in which gaseous flammable substances are formed;
Pre-flame in the gas phase, in which a mixture with an oxidizer is formed;
Flame, or reaction zone in the gas phase, in which the products of pyrolysis are converted into gaseous combustion products;
Combustion products.
The rate of oxygen supply to the combustion zone depends on its diffusion through the combustion product.
In general, since the rate of the chemical reaction in the combustion zone in the types of combustion under consideration depends on the rate of entry of the reacting components and the flame surface through molecular or kinetic diffusion, this type of combustion is called diffusion.
The structure of the diffusion combustion flame consists of three zones (Fig. 18):
Zone 1 contains gases or vapors. There is no combustion in this zone. The temperature does not exceed 500 0 C. Decomposition, pyrolysis of volatiles and heating to the auto-ignition temperature occurs.
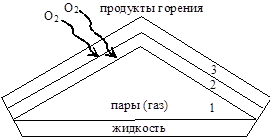
Rice. 18. Flame structure.
In zone 2, a mixture of vapors (gases) with atmospheric oxygen is formed and incomplete combustion occurs to CO with partial reduction to carbon (little oxygen):
C n H m + O 2 → CO + CO 2 + H 2 O;
In the 3rd external zone, complete combustion of the products of the second zone occurs and the maximum flame temperature is observed:
2CO+O 2 =2CO 2 ;
The flame height is proportional to the diffusion coefficient and gas flow rate and inversely proportional to the gas density.
All types of diffusion combustion are inherent in fires.
Kinetic Combustion is the combustion of pre-mixed flammable gas, steam or dust with an oxidizer. In this case, the burning rate depends only on the physicochemical properties of the combustible mixture (thermal conductivity, heat capacity, turbulence, concentration of substances, pressure, etc.). Therefore, the burning rate increases sharply. This type of combustion is inherent in explosions.
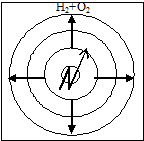 In this case, when the combustible mixture is ignited at any point, the flame front moves from the combustion products into the fresh mixture. Thus, the flame during kinetic combustion is most often unsteady (Fig. 19).
In this case, when the combustible mixture is ignited at any point, the flame front moves from the combustion products into the fresh mixture. Thus, the flame during kinetic combustion is most often unsteady (Fig. 19).
Rice. 19. Scheme of flame propagation in a combustible mixture: - ignition source; - direction of movement of the flame front.
Although, if you mix it first flammable gas with air and feed it into the burner, then upon ignition a stationary flame is formed, provided that the flow rate of the mixture is equal to the speed of flame propagation.
If the gas supply speed is increased, the flame breaks away from the burner and may go out. And if the speed is reduced, the flame will be drawn into the burner with a possible explosion.
According to combustion degree, i.e. completeness of the combustion reaction to the final products, combustion occurs complete and incomplete.
So in zone 2 (Fig. 18) combustion is incomplete, because There is insufficient oxygen supply, which is partially consumed in zone 3, and intermediate products are formed. The latter burn out in zone 3, where there is more oxygen, until complete combustion. The presence of soot in the smoke indicates incomplete combustion.
Another example: when there is a lack of oxygen, carbon burns to carbon monoxide:
If you add O, then the reaction goes to completion:
2СО+O 2 =2СО 2.
The burning rate depends on the nature of the movement of gases. Therefore, a distinction is made between laminar and turbulent combustion.
Thus, an example of laminar combustion is a candle flame in still air. At laminar combustion layers of gases flow in parallel, without swirling.
Turbulent combustion– vortex movement of gases, in which combustion gases are intensively mixed and the flame front is blurred. The boundary between these types is the Reynolds criterion, which characterizes the relationship between inertial forces and friction forces in the flow:
Where: u- gas flow speed;
n- kinetic viscosity;
l– characteristic linear size.
The Reynolds number at which the transition of a laminar boundary layer to a turbulent one occurs is called critical Re cr, Re cr ~ 2320.
Turbulence increases the combustion rate due to more intense heat transfer from combustion products to the fresh mixture.
4.4. Normal combustion.
Depending on the speed of flame propagation during kinetic combustion, either normal combustion (within a few m/s), or explosive deflagration (tens of m/s), or detonation (thousands of m/s) can occur. These types of combustion can transform into each other.
Normal combustion– this is combustion in which the spread of flame occurs in the absence of external disturbances (turbulence or changes in gas pressure). It depends only on the nature of the flammable substance, i.e. thermal effect, thermal conductivity and diffusion coefficients. Therefore, it is a physical constant of a mixture of a certain composition. In this case, the burning speed is usually 0.3-3.0 m/s. Combustion is called normal because the velocity vector of its propagation is perpendicular to the flame front.
4.5. Deflagration (explosive) combustion.
Normal combustion is unstable and tends to self-accelerate in a closed space. The reason for this is the curvature of the flame front due to friction of the gas against the walls of the vessel and changes in pressure in the mixture.
Let's consider the process of flame propagation in a pipe (Fig. 20).
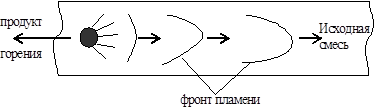
Rice. 20. Scheme of the occurrence of explosive combustion.
At first, at the open end of the pipe, the flame spreads at normal speed, because combustion products expand freely and come out. The pressure of the mixture does not change. The duration of uniform flame propagation depends on the diameter of the pipe, the type of fuel and its concentration.
As the flame front moves inside the pipe, the reaction products, having a larger volume compared to the initial mixture, do not have time to escape outside and their pressure increases. This pressure begins to push in all directions, and therefore, ahead of the flame front, the initial mixture begins to move towards the spread of the flame. The layers adjacent to the walls are inhibited. The flame has the highest speed in the center of the pipe, and the slowest speed is near the walls (due to heat removal in them). Therefore, the flame front extends in the direction of flame propagation, and its surface increases. In proportion to this, the amount of combustible mixture per unit time increases, which entails an increase in pressure, and this, in turn, increases the speed of gas movement, etc. Thus, there is an avalanche-like increase in the speed of flame propagation to hundreds of meters per second.
The process of flame propagation through a combustible gas mixture, in which the self-accelerating combustion reaction spreads due to heating by thermal conduction from the adjacent layer of reaction products, is called deflagration. Typically, deflagration combustion rates are subsonic, i.e. less than 333 m/s.
4.6. Detonation combustion.
If we consider the combustion of a combustible mixture layer by layer, then as a result of thermal expansion of the volume of combustion products, each time a compression wave appears ahead of the flame front. Each subsequent wave, moving through a denser medium, catches up with the previous one and is superimposed on it. Gradually these waves combine into one shock wave (Fig. 21).
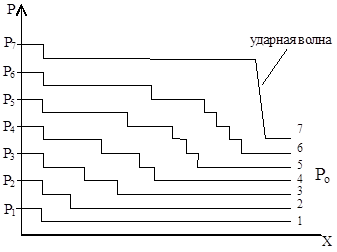
Rice. 21. Scheme of formation of a detonation wave: R o< Р 1 < Р 2 < Р 3 < Р 4 < Р 5 < Р 6 < Р 7 ; 1-7 – нарастание давления в слоях с 1-го по 7-ой.
In a shock wave, as a result of adiabatic compression, the density of gases instantly increases and the temperature rises to T 0 for self-ignition. As a result, the combustible mixture is ignited by a shock wave and detonation– propagation of combustion by ignition by a shock wave. The detonation wave does not go out, because fueled by shock waves from the flame moving behind it.
The peculiarity of detonation is that it occurs at a supersonic speed of 1000-9000 m/s, determined for each mixture composition, and therefore is a physical constant of the mixture. It depends only on the caloric content of the combustible mixture and the heat capacity of the combustion products.
The meeting of a shock wave with an obstacle leads to the formation of a reflected shock wave and even greater pressure.
Detonation is the most dangerous look flame spread, because It has maximum power explosion (N=A/t) and enormous speed. In practice, detonation can be “neutralized” only in the pre-detonation section, i.e. at a distance from the ignition point to the point of detonation combustion. For gases, the length of this section is from 1 to 10 m.
[Previous lecture] [To not see any advertising on the site, you need to become VIP-user.This can be done completely free of charge. Read.
Lecture 14
FIRE SAFETY
1.General information about the combustion process
Basic definitions
Types of combustion
Combustion process
Main indicators of fire hazard of substances
Classification of substances by fire hazard
2. The main sources of fires at the enterprise, during transportation and storage of liquefied gases and hydrocarbons. Grade fire danger industrial enterprises.
3. Classification of production facilities and areas according to fire and explosion hazard
Events for fire prevention. P.p. industrial buildings.
1. General information about the combustion process
Basic definitions
Fire - uncontrolled combustion outside a special fireplace, causing material damage (standard definition).
For people in case of fire dangerous factors are:
open fire, sparks, increased temperature of air and objects;
radiant energy flows, increased environmental temperature, inhalation of hot air, damage and necrosis of the upper respiratory tract
toxic combustion products, smoke, oxygen depletion of air
loss of visibility due to smoke
collapse of buildings and their elements, installations, equipment
Toxic substances formed during a fire are caused by the chemical composition of the burning substance: hair, leather, fabrics, wool - unpleasant-smelling products, cyanide compounds containing soda, aldehydes, ketones, rubber, rubber - isoprene, hydrocarbons, varnishes, products containing neurocelluloid - CO , N 2 O, HCN, Plastics, celluloid - CO, N 2 O, cyanide, formaldehyde, phenol, fluorphosphine, ammonia, acetone, styrene, etc. are highly toxic compounds.
Sunbathing - burning that did not cause material damage.
A person who has received second-degree burns over 30% of their body area has little chance of surviving (without specialized medical care). Time to obtain II degree burns:
26 s at t = 71 C
15c at t = 100С
7s at t= 176С.
Studies conducted in Canada have shown that in a humid environment, typical of a fire, II degree burns are caused by t = 55°C when exposed for 28 s and 70°C for 1 s.
Thus, in a fire in the Invation department store in Brussels, 350 people died and 150 were injured in 10 minutes of fire. During this time, a large department store, occupying an entire hectare, turned into a fire.
1.2. Types of combustion
Combustion - a rapidly occurring chemical reaction (most often oxidation), accompanied by the release of a large amount of heat and usually a bright glow (flame).
Combustion requires the presence of 3 factors:
oxidizing agent (usually O 2, also Cl, F, Br, I, NOX)
flammable substance
source of ignition (i.e. the beginning of the pulse).
Depending on the properties and composition of the flammable substance, the following are distinguished:
A. Homogeneous combustion (same aggregate composition, for example, gases)
B. Heterogeneous combustion (eg solid and liquid).
Depending on the speed of flame propagation, there are:
A. Deflagration (typical of fires)
B. Explosive 100 m/s
B. Detonation 1000 m/s5000 m/s
Depending on the conditions of formation of the combustible mixture:
Diffusion combustion - characterized by the fact that the formation of a combustible mixture occurs during the combustion process as a result of the diffusion of oxygen into the combustion zone. For example, combustion of liquid from an open surface or gases escaping through equipment leaks
Deflagration combustion is diffusion combustion.
Kinetic combustion corresponds to explosive combustion. In this case, the combustible substance and oxygen enter the combustion zone pre-mixed. The determining factor is the rate of the chemical oxidation reaction between the oxidizer and the combustible substance, occurring in the flame front. If the process of kinetic combustion occurs in a closed volume, then the pressure in this volume increases and the temperature of the combustion products increases.
Based on the ratio of fuel and oxidizer, they are divided into:
A. Combustion of lean combustible mixtures (in the subject - an oxidizer, combustion is limited by the connection of the combustible component).
B. Combustion of rich flammable mixtures - accordingly, on the contrary - the fuel limits the content of the oxidizer (contains a humidifier higher than the glass metric ratio of the components).
The occurrence of combustion is associated with the obligatory self-acceleration of the reaction. There are 3 types of self-acceleration:
thermal: subject to the accumulation of heat in the system, the temperature rises, which leads to the acceleration of chemical reactions;
chain: associated with the catalysis of chemical transformations by intermediate reaction products, has special chemical activity (active centers). (i.e., the chemical process does not occur through direct interaction of the original molecules, but with the help of fragments formed during the decay of these molecules).
Real combustion processes are usually carried out by a combined chain-thermal mechanism.
1.3 Types of combustion process
Flash - rapid (almost instantaneous) combustion of flammable mixtures, not accompanied by the formation of compressed gases.
Fire - the occurrence of combustion under the influence of an ignition source (сttignition or spontaneous combustion)
Ignition - fire accompanied by the appearance of a flame.
Spontaneous combustion - a sharp increase in the rate of exothermic reactions leading to combustion of a substance (mixture) in the absence of an ignition source. This can also occur at ambient temperatureignition temperature. This possibility is due to the tendency of substances to oxidize and the conditions for the accumulation of heat released during oxidation. Thus, during spontaneous combustion there is, as it were, an internal impulse.
Depending on the impulse, spontaneous combustion processes are divided into:
thermal,
microbiological,
chemical.
Thermal spontaneous combustion/spontaneous combustion occurs as a result of prolonged exposure to an insignificant heat source. In this case, the substances decompose, are adsorbed and, as a result of oxidative processes, spontaneously ignite. So at temperature100С they are prone to spontaneous combustion sawdust, fiberboard, parquet.
Chemical Spontaneous combustion/spontaneous combustion occurs from exposure of substances to oxygen in air, water, or from the interaction of substances. (Fires from spontaneous combustion of oily rags, overalls, cotton wool, and sometimes even metal shavings).
The tendency of an oil or fat to spontaneous combustion can be judged by its iodine number (the amount of I2 absorbed by 100 g of the oil or fat being tested).
The higher the iodine number, the lower the spontaneous combustion temperature, the more dangerous the substance.
Microbiological spontaneous combustion - at appropriate humidity and temperature in plant products with the intensification of the vital activity of organisms (a fungus is formed - the so-called cobweb litharge), which causes an increase in temperature.
(To prevent - regular monitoring of temperature and humidity, limiting humidity and temperature
Self-ignition - spontaneous combustion accompanied by the appearance of a flame.
Explosion - an extremely rapid chemical transformation, accompanied by the release of energy and compressed gases capable of producing work.
Detonation - heat transfer from layer to layer occurs due to the propagation of a shock wave.
When assessing the fire hazard of substances, it is necessary to take into account their state of aggregation.
Since combustion usually occurs in gas environment, as indicators of fire safety (FS), it is necessary to take into account the conditions under which a sufficient amount of gaseous products is formed for combustion.




